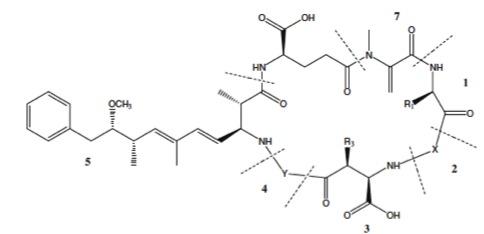
Rapid Opioid Identification and Quantitation by Paper Spray Mass Spectrometry (PS-MS)

Problematic substance use, and associated harms such as overdose, is a very complicated, multi-faceted issue. Since a public health emergency was declared in British Columbia in April 2016, more than 2600 British Columbians have died from illicit drug overdoses, and opioid-related fatalities in BC are currently (as of April 2018) almost triple (29 per 100,000) the per capita rate for the rest of the country (10.9 per 100,000). There is little evidence of a declining trend in fatal overdose victims, with a predominantly male demographic (81%), mainly between the ages of 29-59 years. The Indigenous population is over-represented in the demographic of drug overdose fatalities, with members 3 times more likely to die when they do experience an overdose event. Indigenous women are at much higher risk of overdose death than non-Indigenous women, with an even gender split in the BC Indigenous population. Within this arena, the development of easy to use, rapid, and importantly quantitative drug checking strategies could provide a key tool for addiction medicine and harm reduction specialists. Current on-site drug checking strategies involve 'yes/no' tests, or suffer from inadequate specificity and/or sensitivity for the myriad of potential toxic drugs that may be present on the street. We are actively developing PS-MS analysis approaches that yield high sensitivity, quantitative and accurate measurement of trace and major component drugs in less than a minute. The strategy is very successful in the laboratory, and we are seeking funding to bring it into clinical trials for opioid harm reduction in BC and beyond.
Quantitative Microcystin Measurements via CP-MIMS
Microcystins (MCs) are a potent cla
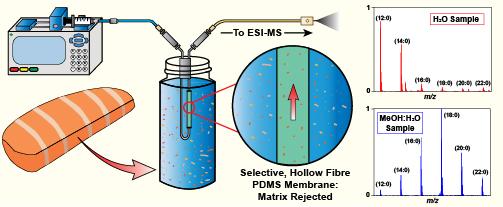
Long Chain Fatty Acid Quantitation: CP-MIMS with Modified Donor Phases
CP-MIMS Utilizing Direct Liquid Electron Ionization (CP-MIMS-LEI)
Condensed-Phase Membrane Introduction Mass Spectrometry (CP-MIMS)

We have pioneered the use of mass spectrometry coupled to membrane interfaces to study intrinsic molecular and material properties at low permeant concentrations and in multi-component mixtures. This contribution opens significant new capacity in the study of mass transport in semi-permeable membranes. Furthermore, understanding the factors that limit membrane transport has directly contributed to the evolution of an important MIMS variant (known as Condensed Phase MIMS or CP-MIMS) in our group. This invention, protected by Canada, US and worldwide (PCT) patents, uses a liquid (condensed) acceptor phase for analyte transfer. CP-MIMS has already had a significant impact on the field of environmental chemistry, opening new territory by widening the analyte range to include low volatility targets of concern such as polyaromatic hydrocarbons (PAHs), pesticides, surfactants and drugs, leading to substantial progress and new applications.
The characterization of this technique was followed up with a paper that described improved performance characteristics for thinner membranes and their application to pharmaceutical compounds and naphthenic acids. This was the first paper to establish a semi-quantitative rapid screening method for multiple naphthenic acid isomer classes directly in complex oil sands process water samples using an immersion probe designed in our group. The impact of CP-MIMS is substantial, enabling the chemical determination of a much broader range of environmentally and biologically important chemicals of concern, attracting the interest of industries in the energy sector, regulators and analytical service providers. In-situ reaction monitoring by dynamic chemical systems by MIMS Our group has employed the continuous real-time capabilities of MIMS to investigate the transformation of organic compounds directly in aqueous solution. We published the first example of an in-situ sampling platform to extract rate constants by simultaneously monitoring the degradation of individual gasoline components by tandem MS techniques at parts per trillion levels. We have extended this work to monitor the kinetics of photosensitized halogenation reactions of trace halocarbon contaminants directly in natural waters containing dissolved organic matter as well as the formation kinetics of volatile and non-volatile disinfection by-products. We have applied this approach to monitor dynamic changes in both the distribution and quantity of naphthenic acids in oil sands processed waters under the influence of ultraviolet light and adsorbents. The sensitivity and selectivity afforded by tandem MS strategies coupled to the direct measurement of reaction kinetics in complex samples without laborious and time-consuming handling has significantly improved our ability to follow natural and industrial processes, including remediation and treatment efforts. Our work in this area is important to the field of environmental organic chemistry, enabling kinetic studies of chemical transformations directly in environmental media. This contribution addresses a long-standing problem in the investigation of environmental transformations- that of extrapolating measurements made in-silico under controlled conditions to environmental systems, such as lakes, estuaries and tailing ponds.
Field portable MS systems with real-time geospatial mapping

We subsequently added adaptive feedback and real-time, cloud based data storage and analysis. Interactive results in the Google Earth™ web environment enabled, for the first time, rapid access to quantitative field MS measurements in an intuitive format. The time series data can be viewed in real-time and/or interrogated later providing unprecedented data sets that can be linked to sources, human exposure and health effects. This significant development removes delays between sampling/analysis and informed, results-based decisions. Rapid response is key in critical events such as spills or accidents, and allows intelligent, adaptive decisions for field sampling (site selection, prescreening) prior to off-line lab, based measurements. MIMS provides ‘real-time’, free analyte concentrations, and temporal concentration changes (e.g. moving emission plumes) can be measured, recording transient events that may have significant health implications. Our leadership in this area has helped to inspire academics and industry to develop MIMS monitoring systems capable of direct, in-field monitoring. Real-time, cloud based data handling is now becoming more common for field measurements by MS and other methods. This pioneering work has the potential to revolutionize environmental assessments and source apportionment studies and has attracted considerable attention from both industrial users and public agencies. This work represents a significant milestone in the evolution of environmental analysis from the lab to the field. This research was funded by Statoil Hydro, Norway.
Membrane Interface Design Development
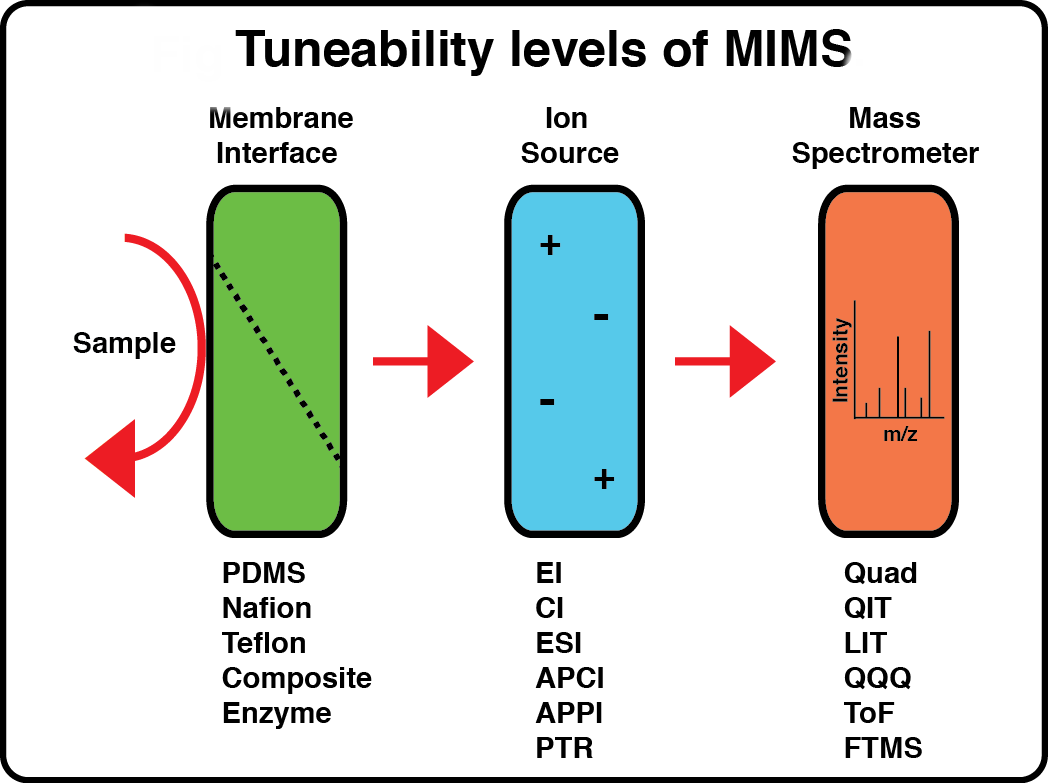

MIMS membranes perform a direct, online, non-exhaustive extraction of permeating analytes, obviating contamination, dilution and recovery losses associated with off-line sample preparation, while simultaneously rejecting the bulk sample matrix and allowing sample remeasurement. Our research group aims to improve MIMS membrane transport, develop small volume, microfluidic based interfaces and couple them with flexible ionization approaches.We have used several interface variants for continuous, online sampling systems, exploiting hollow fibre membranes in robust flow cell geometries and direct insertion/immersion probes. Micro to nano flow scale MIMS systems based on hollow fibre membranes will be further developed because of their easy incorporation with conventional (HPLC) fluid handling. Microfluidics adoption is a better approach, enabling new applications for very small samples (blood spots, cerebrospinal fluids) and in situ diagnostics. Stereolithographic 3D printing will be used to make miniature, sheet membrane based, microfluidic MIMS interface systems.
Better understanding membrane transport behavior and the increased selectivity/selectivity it affords opens new territory. Small sample measurements with simple, one use interfaces and multiplexed, automated microfluidic systems will elevate MIMS to a realistic alternative for high throughput, direct environmental and bioanalytical screening with continuous measurement capabilities.
Correlation of HPLC-HRMS separation and speciation of naphthenic acids to direct sampling, on-line membrane introduction mass spectrometry techniques
Napthenic Acids (NAs), a by-pro

